1 chlorobutane boiling point
Home » datasheet » 1 chlorobutane boiling point1 chlorobutane boiling point
1 Chlorobutane Boiling Point. As the vapors gradually. We call a substituent that contains one less hydrogen than the corresponding alkane an alkyl group. The name of an alkyl group is obtained by dropping the suffix -ane of the. Ethanol is a primary alcohol that is ethane in which one of the hydrogens is substituted by a hydroxy group.
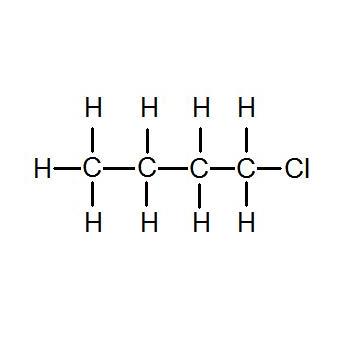
Before finding this i was looking through my class notes and textbook trying to find out what made a difference in a molecules boiling point and i couldnt find a good answerusing this page i was able to answer a question on my. Hexane boils at a higher temperature than heptane. We call a substituent that contains one less hydrogen than the corresponding alkane an alkyl group. 1-Chlorobutane 998 for HPLC. Iii It is due to I effect of halogens it deactivates benzene ring towards electrophilic substitution reactions. A strong signal at 1700.
How much sucrose is to be added to 500 g of water such that it boils at 100CMolal elevation constant for water is 052 K kg mol -1.
Boiling point increases with increase in molecular mass of halogen atom for the similar type of alkyl halide. Thus the boiling point of isopropyl alcohol is lower than that of 1-chloropropane. Further the boiling point decreases with an increase in branching in the chain. Hexane boils at a higher temperature than heptane. Which bond angles are present in the molecule ethane. 1-Chlorobutane 0886 Tetrahydrofuran 0889 Ethyl acetate 0895 o-Xylene 0897 Hexamethylphosphorus triamide 0898 2-Ethoxyethyl ether 0909 NN-Dimethylacetamide 0937 Diethylene glycol dimethyl ether.
Source: chemspider.com
This is because with the. Boiling point and concentrated HCl volume mmoles equivalents Volume mL Mass g mmoles Density Equiv Boiling PointProperties tert-pentyl alcohol 60 48 55 0805 10 Bp102 Concentrated 45 55 12 10 Extremely. Isopropyl chloride 1. Chlorobutane 1-Chlorure de butyle French CAS-109-69-3. Butane boils at a higher temperature than propane.
Source: merckmillipore.com
Thus the boiling point of isopropyl alcohol is lower than that of 1-chloropropane. Which is the correct increasing order of boiling points of the following compounds. The examples show that the boiling points fall as the isomers go from a primary to a secondary to a tertiary halogenoalkane. The boiling point of methanol is 65 degrees Celsius and the boiling point of ethanol is 78 degrees Celsius. The increasing order of boiling point is.
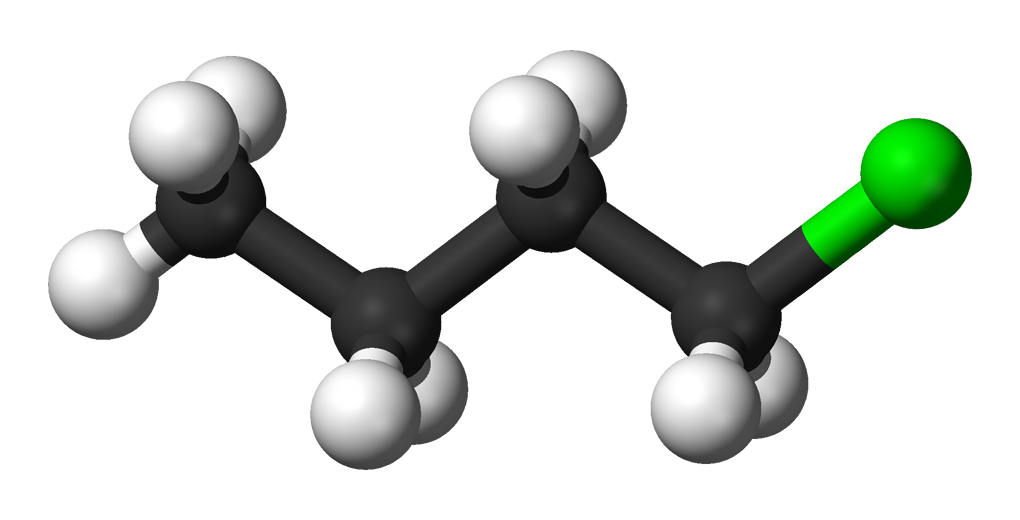 Source: en.wikipedia.org
Source: en.wikipedia.org
You may recall that boiling point is a function of intermolecular interactions which was discussed in the chapter on solutions and colloids. Boiling point started to make sense already by that list at the beginning. Jan 20 2021 Lampen was up about 4. Thus the boiling point of isopropyl alcohol is lower than that of 1-chloropropane. 4 min read Was this answer.

Further the boiling point decreases with an increase in branching in the chain. 0943 NN-Dimethylformamide 0944 2. In S N 2 mechanism reactivity depends upon the steric hindrance around the C-atom. Further the boiling point decreases with an increase in branching in the chain. The examples show that the boiling points fall as the isomers go from a primary to a secondary to a tertiary halogenoalkane.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
It has a role as an antiseptic drug a polar solvent a neurotoxin a central nervous system depressant a teratogenic agent a NMDA receptor antagonist a protein kinase C agonist a disinfectant a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite. 1 - Iodobutane 1 - Bromobutane 1 - Chlorobutane Butane. Index of refraction 14. Boiling Point 8160 C at 1 atm. Ii 2-Bromobutane is chiral therefore optically active whereas 1 -chlorobutane is not chiral therefore optically inactive.
Academiaedu is a platform for academics to share research papers. Because of these the vander Waals force of attraction between the molecule decreases and consequently boiling point decreases. Butane has no halogen atom and rest of all. Which is the correct increasing order of boiling points of the following compounds. Draw 3 dimensional structures of all cyclohexanes having a methyl and a chloro substituent.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Which bond angles are present in the molecule ethane. One of the products of the reaction between hexane and bromine is shown below. 0943 NN-Dimethylformamide 0944 2. Haloalkanes and Haloarenes Previous Year Question 11. As molecular mass is increasing so boiling point also increase.
 Source: molinstincts.com
Source: molinstincts.com
We call a substituent that contains one less hydrogen than the corresponding alkane an alkyl group. In S N 2 mechanism reactivity depends upon the steric hindrance around the C-atom. It has a role as an antiseptic drug a polar solvent a neurotoxin a central nervous system depressant a teratogenic agent a NMDA receptor antagonist a protein kinase C agonist a disinfectant a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite. A strong signal at 1700. Because of these the vander Waals force of attraction between the molecule decreases and consequently boiling point decreases.
 Source: chemsrc.com
Source: chemsrc.com
1-Chlorobutane 995 anhydrous AcroSealR EINECS 203-696-6. Which statement or statements about the boiling point of alkanes are true. Chlorobutane 1-Chlorure de butyle French CAS-109-69-3. C5h11cl isomers - egzotyka-lastminutepl. A The higher the surface area the higher will be the intermolecular forces of attraction and thus boiling point too.
Source: stenutz.eu
D Butane 1-Chlorobutane 1-Iodobutane 1-Bromobutane Solution. Which bond angles are present in the molecule ethane. You may recall that boiling point is a function of intermolecular interactions which was discussed in the chapter on solutions and colloids. Which is the correct increasing order of boiling points of the following compounds. Rank the following 3 compounds in terms of increasing boiling point.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title 1 chlorobutane boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.