1 chloro 2 methylpropane boiling point
Home » datasheet » 1 chloro 2 methylpropane boiling point1 chloro 2 methylpropane boiling point
1 Chloro 2 Methylpropane Boiling Point. Also people ask about. The main target users are workers and those responsible for occupational safety and health. Wir beraten Sie gerne. 1 Structures Expand this section.
 2 Chloro 2 Methylpropane 507 20 0 Tokyo Chemical Industry Uk Ltd From tcichemicals.com
2 Chloro 2 Methylpropane 507 20 0 Tokyo Chemical Industry Uk Ltd From tcichemicals.com
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. Density 65 lb gal. Vapors are heavier than air. 1 Melting Point -457 C 2 Density 077674 gcm 3 at 25C 3 Vapor Density 143 relative air1 Coefficient of Thermal Expansion 0001397 C-1 4 Refractive Index 13442 Na D at 20C 5 Viscosity 0352 cP at 20C 6 Triple Point -438 C 7 Critical Temperature 2724 C 8 Critical Pressure 483 MPa 8 Critical Volume 0173 L Surface Tension 2929 dynecm at. The main target users are workers and those responsible for occupational safety and health. C5h11cl isomers - egzotyka-lastminutepl.
2 Names and Identifiers Expand this section.
The ICSC project is a common undertaking between the World Health Organization WHO and. These 3 commercially available compounds have molecular formula C4H9F. From the straight-chain alkane we get 1-Chlorobutane and 2-Chlorobutane From the branched-chain alkane we get 1-Chloro-2-methylpropane and 2-Chloro-2 Another example of constitutional isomers is 12-dibromocyclohexane and 14-dibromocyclohexane. The main target users are workers and those responsible for occupational safety and health. Boiling Point 8160 C at 1 atm. Analytical 4 ACS reagent 2 BioReagent 2 Technique.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Also people ask about. So the boiling point of this compound should be relatively high because it has a large surface area and it is a polar molecule so there are dipole -dipole forces present. Vapors are heavier than air. Density 65 lb gal. Now take a alcohol that is a shorter chain such as propanol.
 Source: tcichemicals.com
Source: tcichemicals.com
Density 65 lb gal. 1 Structures Expand this section. Dui ruined my life reddit. Alcohol should have a higher boiling point because it has a hygrogen bond. Density 65 lb gal.
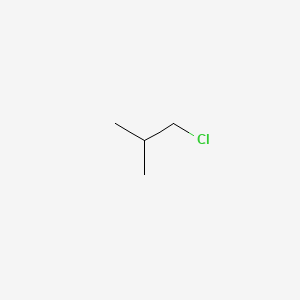
Boiling Point C Feature. 4 Spectral Information Expand this section. Isomers of c4h9f email protected 371 67881775. So the boiling point of this compound should be relatively high because it has a large surface area and it is a polar molecule so there are dipole -dipole forces present. Also people ask about.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products. So the boiling point of this compound should be relatively high because it has a large surface area and it is a polar molecule so there are dipole -dipole forces present. Vapors are heavier than air. Ethanol with a small amount of an adulterant added so as to be unfit for use as a beverage. Klicken Sie auf das Bild oder hier um unsere Gase-Liste zu öffnen Wir beraten Sie gerne um Ihnen die bestmögliche Lösung zu.
 Source: tcichemicals.com
Source: tcichemicals.com
Vapors are heavier than air. Sort by Relevance. 3 Chemical and Physical Properties Expand this section. Analytical 4 ACS reagent 2 BioReagent 2 Technique. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
 Source: chemnet.com
Source: chemnet.com
The primary aim of the cards is to promote the safe use of chemicals in the workplace. Alcohol should have a higher boiling point because it has a hygrogen bond. The main target users are workers and those responsible for occupational safety and health. These 3 commercially available compounds have molecular formula C4H9F. The ICSC project is a common undertaking between the World Health Organization WHO and.
Wir beraten Sie gerne. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. From the straight-chain alkane we get 1-Chlorobutane and 2-Chlorobutane From the branched-chain alkane we get 1-Chloro-2-methylpropane and 2-Chloro-2 Another example of constitutional isomers is 12-dibromocyclohexane and 14-dibromocyclohexane. So the boiling point of this compound should be relatively high because it has a large surface area and it is a polar molecule so there are dipole -dipole forces present.
 Source: fishersci.se
Source: fishersci.se
Ethanol with a small amount of an adulterant added so as to be unfit for use as a beverage. 3 Chemical and Physical Properties Expand this section. 1 Structures Expand this section. Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products. So the boiling point of this compound should be relatively high because it has a large surface area and it is a polar molecule so there are dipole -dipole forces present.
Source: chemspider.com
But the propanol molecule is a much shorter chain so it has a smaller surface area than. From the straight-chain alkane we get 1-Chlorobutane and 2-Chlorobutane From the branched-chain alkane we get 1-Chloro-2-methylpropane and 2-Chloro-2 Another example of constitutional isomers is 12-dibromocyclohexane and 14-dibromocyclohexane. So the boiling point of this compound should be relatively high because it has a large surface area and it is a polar molecule so there are dipole -dipole forces present. Boiling Point 8160 C at 1 atm. 1 Melting Point -457 C 2 Density 077674 gcm 3 at 25C 3 Vapor Density 143 relative air1 Coefficient of Thermal Expansion 0001397 C-1 4 Refractive Index 13442 Na D at 20C 5 Viscosity 0352 cP at 20C 6 Triple Point -438 C 7 Critical Temperature 2724 C 8 Critical Pressure 483 MPa 8 Critical Volume 0173 L Surface Tension 2929 dynecm at.
 Source: fishersci.fi
Source: fishersci.fi
C5h11cl isomers - egzotyka-lastminutepl. 1 Melting Point -457 C 2 Density 077674 gcm 3 at 25C 3 Vapor Density 143 relative air1 Coefficient of Thermal Expansion 0001397 C-1 4 Refractive Index 13442 Na D at 20C 5 Viscosity 0352 cP at 20C 6 Triple Point -438 C 7 Critical Temperature 2724 C 8 Critical Pressure 483 MPa 8 Critical Volume 0173 L Surface Tension 2929 dynecm at. 3 Chemical and Physical Properties Expand this section. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. These 3 commercially available compounds have molecular formula C4H9F.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title 1 chloro 2 methylpropane boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.